Preventing COVID-19 transmission into the workplace is now among the highest priorities for all company executives and it’s widely accepted that testing is key to the global exit strategy from the disease.
Martek Lifecare (Martek), part of James Fisher and Sons plc, has launched COVID-19 occupational screening, combining antigen and antibody testing.
Both kinds of tests help employers make decisions about measures to prevent an outbreak and inform critical return to work decisions.
Martek’s Antigen tests screen directly for the virus itself providing soonest possible detection.
Samples are taken using swabs from the nose and throat and are sent to a UKAS accredited laboratory for machine processing, delivering results within two days.
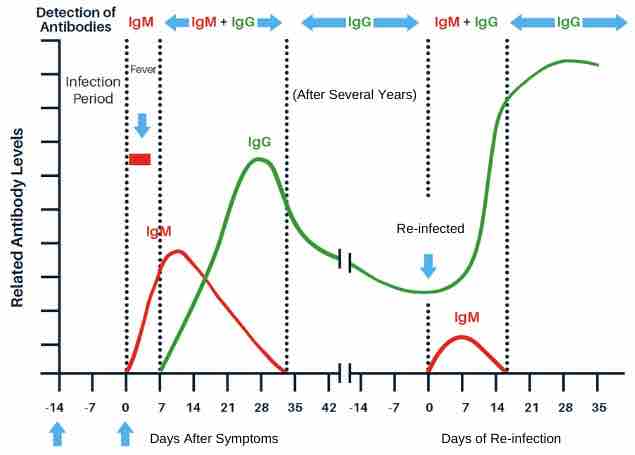
By implementing a combined antibody and antigen test protocol, esteemed medical opinion reports a 98.6% detection rate within the first 5.5 days of infection1.
Infected employees without symptoms pose the biggest risk to employers and an article in the British Medical Journal3 reports that four fifths of cases are asymptomatic.
According to McKinsey2, studies in China and Singapore report that asymptomatic transmission may account for ~50% of cases.
Such employees left undiagnosed (ie, without pre- screening) would potentially spread the infection throughout the workplace, while being totally oblivious.
Antibody tests are the most effective and appropriate way to diagnose this category of subject, with 97% clinical specificity. Research by McKinsey2 concludes that, “Antibody tests are currently the best method for detecting asymptomatic cases”.
Martek’s CoronaSafe antibody test is approved and certified by the European Union as meeting the safety, quality and performance requirements of Standard IVDD 98/79/EC – the highest standard applicable for these devices. It’s also in wide scale use by authorities/corporations in Japan, China, Germany, Poland, Indonesia and is formally certified by the China Food and Drug Administration, Philippines Food and Drug Administration and the Singapore Health Sciences Authority.
Martek’s CEO Paul Luen commented: “Screening employees through a regimen of combined antigen and antibody testing is a critical step to prevent avoidable transmission from asymptomatic employees while informing critical return to work decisions.”
References:
1. Expert comment on testing, Dr James Gill: https://www.sciencemediacentre.org/expert-comments-on-different-types-of-test-for-covid-19/
2. McKinsey Covid-19 Briefing: https://www.mckinsey.com/~/media/mckinsey/business%20functions/risk/our%20insights/covid%2019%20implications%20for%20business/covid%2019%20march%2025/covid-19-facts-and-insights-march-25-v3.ashx
3. British Medical Journal article: https://www.bmj.com/content/369/bmj.m1375
The tests are further certified to the following additional International Standards:
- ISO 14971 Standard for the application of risk management to medical devices.
- ISO 13485 Medical Devices QMS certified manufacturer.
- EN 13975 Requirements for acceptance testing of finished in vitro diagnostic medical device.
- ISO 18113-2 Requirements for information of in vitro diagnostic (IVD) reagents for professional use.
- ISO 18113-4 Requirements for information of in vitro diagnostic (IVD) reagents for self-testing.
- BS EN 13612:2002 Performance evaluation of in vitro diagnostic medical devices (IVD MDs for self-testing.
 Engineer News Network The ultimate online news and information resource for today’s engineer
Engineer News Network The ultimate online news and information resource for today’s engineer