Martin Gadsby and Ernie Hillier look at how to use Process Analytical Technology (PAT) to improve material management in flow chemistry applications
Continuous flow chemistry processes can greatly benefit from a Process Analytical Technology (PAT) framework that incorporates material tracking and tracing functions. In particular, when commercial-scale continuous processes are conducted in a GMP (Good Manufacturing Practices) compliant environment, information and knowledge about the movement of products is critical for quality and regulatory assurance purposes.
In these applications, a continuous track and trace system must be able to track the movement of products as well as take into account the forward and back mixing that occurs in many continuous processes. Ultimately, the solution should be able to make accurate quality predictions based on mathematical models and empirical data.
Running chemical reactions in a continuous flow stream, rather than in conventional batches where materials are weighed and added to a batch unit operation, can deliver significant benefits to pharmaceutical manufacturers. Continuous flow chemistry applications can considerably shorten cycle times and costs as well as improve productivity, efficiency and throughput. At the same time, they enhance end product quality and consistency by using PAT-driven quality assurance strategies. These applications can slash the downtime associated with off-line quality control and testing, plus offer real-time monitoring capabilities.
While continuous processes offer a robust framework to help pharmaceutical companies deliver high-quality products more efficiently, the IT and automation infrastructure needs to provide complete visibility associated with the movement of raw materials throughout manufacturing in order to meet regulatory compliance and GMP (1). More precisely, it is crucial for pharmaceutical producers to be able to track the raw material lots that they use from process input to final batches. In other words, they must be able to identify with a high degree of confidence, the location of any material at any given time.
In addition to lot to end product traceability, the quality assurance data sets of the in-flight materials that have been measured by a PAT knowledge manager have to be tracked through the process and associated with the final batches. In this way, for each finished product it is possible to have complete sets of quality assurance data, together with information on the contained raw material lots. Yet further, the tracking system has to monitor the movement of any suspect or out of specification material as it progresses through the system, from the detection point to the sampling or rejection point. Also, it needs to drive the process valves or diverting system to acquire the target product. To achieve this level of functionality, the track and trace system requires real-time operation together with real world connectivity.
Since manufacturing using flow chemistry is extremely different from batch production operations, they need alternative methods to ensure material track- and traceability. Traditional batch tracking solutions generally rely on the discrete, transactional movement of product batches or sub-batches where mixing between these units seldom occur. Thus, such track and trace systems are relatively straightforward to implement, and the movement of product can be defined with total confidence. In continuous applications a constant flow of materials that change state and structure travel through the various unit operations with varying levels of forward and back mixing. Therefore, the product movement is very much non-discrete and it is not possible to use conventional methods to track product – the tracking system is very different and it is a common mistake to think that they are interchangeable. Various track and trace methods and approaches can be taken to solve the problem, however no matter what solution is chosen, the undeniable truth is that the tracking algorithm will be based on probability. In a batch system it is arguably possible to trace, with total confidence the movement of any specific entity from one unit operation to the next, however with continuous processes this is impossible irrespective of whether you use mechanistic, empirical or hybrid modelling. Thus businesses need to make sure that the levels of probability are acceptable in practical applications.
Effective material track and trace for continuous flow
To address these challenges and successfully adopt flow chemistry on pharmaceutical production lines, manufacturers need to select a tracking solution that is flexible, easy to configure and provides real-time control and process status visibility. One key element to look for is the ability of a system to effectively interact with PAT and automation systems in order to leverage the insight provided by real-time in-process data. This aspect is crucial for the system to dynamically respond to the prevailing process conditions and, in turn, provide timely feedback to operators and automation systems on in-flight product quality, process conditions and the movement of product so as to enable the optimisation of product quality and process productivity. Where necessary, products for sampling or rejection can be removed from the line. This must be done while ensuring that only products meeting the quality criteria are output from the process. This combined automation, PAT and tracking configuration maximises the efficiency and quality of production whilst also ensuring GMP and regulatory requirements.
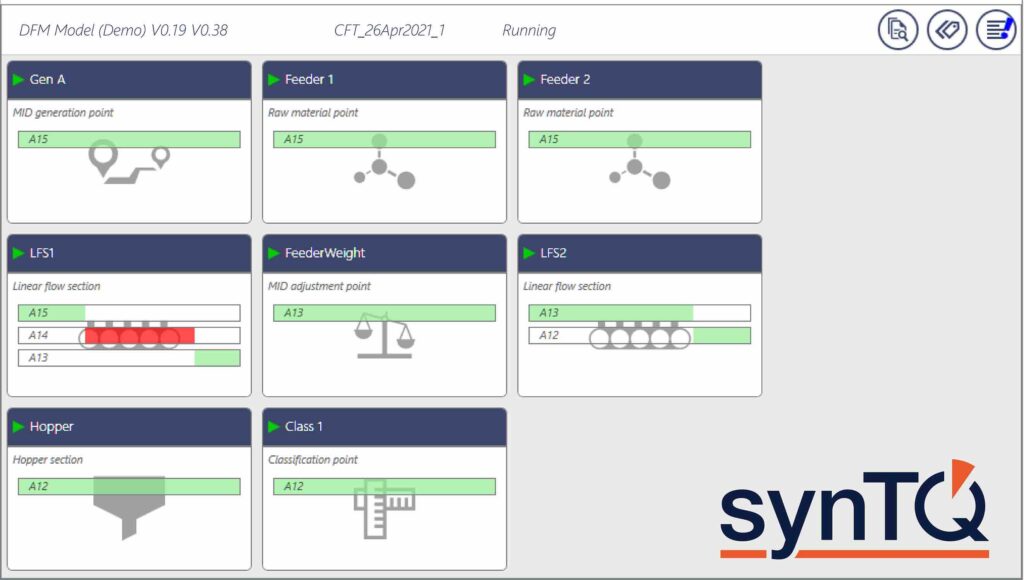
An example of an effective system to increase supply chain visibility is the synTQ Dynamic Flow Modeller (DFM) by Optimal, used in conjunction with the company’ PAT knowledge management platform, synTQ. This combines a material tracking system with a PAT data manager, which collects, processes, fuses and stores analytical information as well as predictions while communicating with the entire PAT environment. synTQ DFM provides a straightforward and practical method to track products for sampling, rejection and to trace products, as well as their associated quality data, from starting lots to finished batches. This is achieved by configuring an off-line, ‘in silico’ model of production lines and then in real-time, by integrating synTQ DFM into the control, automation and PAT architectures.
Significant advantages are possible if the PAT platform can incorporate technologies to incorporate the real-time detection of low-concentration impurities. This is achieved, for instance, by communicating with highly accurate on-line analysers, such as gas chromatographs (GC) or high-performance liquid chromatographs (HPLC), which run continuously but in single-injection mode. Depending on the attribute being measured, the process reaction may be ‘steered’ to ensure that the creation of any unwanted, low concentration by-product is minimised, and in the worst case scenario, material with too high a level of unwanted trace materials can be rejected from the system.
Choose practicality and convenience
When looking at the features of a material track and trace solution, businesses need to take a number of practical considerations into account. In effect, they should favour a PAT knowledge management system that is cost-effective as well as easy to use, implement and maintain. This will improve return on investment (ROI) while supporting operators in their daily tasks. In practice, this means selecting a platform that requires only minimal configuration, with no coding. As a result, pharmaceutical manufacturers can benefit from an intuitive software solution that can be set up quickly and is easily adjusted, edited or updated by authorised staff who do not need programming skills.
In addition, companies should consider the cost/benefit advantages of using a process technique that relies in part on mechanistic modelling together with empirical modelling versus an approach that relies on empirical modelling only. Both have their place, and whilst a mechanistic approach has advantages, the current state of the art means that pure mechanistic modelling is seldom, if ever, possible. Invariably, empirically-derived data and parameterisation will be used together with the mechanistic model to create a hybrid model that may be declared as mechanistic, but is in fact not truly so. Purely empirical modelling, on the other hand, can be quicker to establish and set up. While practical testing is likely to be necessary for an empirical model, very often such practical experimentation is needed for parameterisation of ‘mechanistic’ approaches too. Thus, an empirical system can in fact be faster and more cost-effective to implement.
Finally, when choosing a forward-looking solution, businesses should consider the use of cost effective, practical software that has adopted qualitative, empirical dynamic flow models as a backbone, rather than a more expensive and complex mechanistic flowsheet solution.
Conclusions
Material tracking and tracing is fundamental to creating successful flow chemistry applications in GMP and regulatory compliant applications. Together with a PAT system, they ensure the in-process and final product quality together with being fundamental for the trigging of sampling and rejection in a timely way. Also, they provide lot to final batch traceability and related datasets. This, in turn, provides quality assurance and regulators with the quality as well as track and trace data that is essential for quality assurance and batch release.
Key features to look for in a material tracking and tracing system include offline, flexible model creation; the ability to interact in real time with PAT knowledge management and automation platforms, cost-effectiveness, ease of use and a straightforward path to implementation and validation. By selecting a solution with these characteristics, such as synTQ and synTQ DFM, pharmaceutical manufacturers can advance their processing strategies and transform manufacturing from batch to flow, while minimising the time and capital expenditure (CAPEX) associated with such strategies. Ultimately, an effective and automated material and quality assurance management system can improve a company’s competitiveness and reliability while enhancing product quality and manufacturing sustainability.
References and Notes:
(1) The U.S. Food and Drug Administration. Guidance Document: Quality Considerations for Continuous Manufacturing – Guidance for Industry. Docket Number: FDA-2019-D-0298. DRAFT GUIDANCE (2019).
Martin Gadsby is Director at Optimal Industrial Technologies, and Ernie Hillier is Principal Owner at EJH Consulting.
 Engineer News Network The ultimate online news and information resource for today’s engineer
Engineer News Network The ultimate online news and information resource for today’s engineer